what is the law of conservation of matter
All EPBC web materials are currently being updated in relation to the new matter of national environmental significance. The law of conservation of energy states that the total energy of an isolated system remains constant.

Matter Laws Of Chemical Combinations The Laws Of Chemical Combination Govern How Elements Will Fo Law Of Multiple Proportions Proportions Worksheet Chemistry
In physics and chemistry the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy the mass of the system must remain constant over time as the systems mass cannot change so quantity can neither be added nor be removed.

. It is one of the laws of chemical combinations in chemistry. History of the Law of the Conservation of Mass. In physics a conservation law states that a particular measurable property of an isolated physical system does not change as the system evolves over time. The first kind of energy to be recognized was kinetic energy or energy of motionIn certain particle collisions called elastic the sum of the kinetic energy of the particles before collision is equal to the sum of the kinetic.
According to this law the mass of an object or collection of objects never changes over time no. The law of conservation of matter or principle of matter conservation states that the mass of an object or collection of objects never changes over time no matter how the constituent parts rearrange themselves. The Law of Conservation of Mass Matter is anything that has mass and takes up space. For example when a piece of wood burns the mass of the smoke ashes and gases equals the original mass of the total charcoal and the oxygen when it first reacts.
It includes molecules atoms fundamental particles and any substance that these particles make up. In a closed system the exchange of. Law of Conservation of Matter DEFINE. The law of conservation of energy is a physical law that states energy cannot be created or destroyed but may be changed from one form to another.
According to the law in an isolated system matter cannot be created or destroyed only changed. For example when wood burns the mass of the soot ashes and gases equals the original mass of the charcoal and the oxygen when it. However Antoine Lavoisier described the law of conservation of mass or the principle of massmatter conservation as a fundamental principle of physics in 1789. The later is used in physics while the former in chemistry.
The law of energy conservation applies to all matter and energy in every system no matter what the conditions. Law of conservation of energy is a false idea. Amendments to the EPBC Act became law on 22 June 2013 making water resources a matter of national environmental significance in relation to coal seam gas and large coal mining development. The box is placed in the sun for a year and a tree grows inside the box.
_____ Design an investigation to demonstrate the law of conservation of mass using a seltzer tablet and flask. It is also known as the law of conservation of mass. Scotty wont be beaming you up anytime soon. The law of conservation of matter is a general law in physics and chemistry applicable for any system closed to all transfers of matter and energy.
The law of conservation of mass is a scientific law popularized and systematized by the 18th-century French chemist Antoine Lavoisier. When salt is added to water it forms saltwater. The law takes effect March 1 2020 and. The law of conservation of matter says that in chemical reactions the total mass of the products must equal the total mass of the reactants.
This is the law of conservation of mass. Tim and Moby explain thermodynamics. Inside a large sealed glass box there is plenty of moist soil a water tank with drip irrigation and the seed of a fast growing tree. A US dollar bill weights about 1 gram.
The law of conservation of mass states that any object or particular matter cannot be created or destroyed when it is undergoing a chemical reaction. Another problem which tests students understanding of the theory of conservation of matter is the following. The ancient Greeks first proposed the idea that the total amount of matter in the universe is constant. Tells us that the amount of matter stays the same even when a substance changes form.
Matter can change form through physical and chemical changes but through any of these changes matter is conserved. The law of conservation of matter is a fundamental law in science. Law of Conservation of Mass. A scientific unit of measuring how heavy something is.
Credit for discovering the law may be given to either Mikhail Lomonosov or Antoine Lavoisier. The law has huge applications in chemistry physics and engineering. Conservation law in physics a principle that states that a certain physical property that is a measurable quantity does not change in the course of time within an isolated physical system. Another way of stating this law of chemistry is to say the total energy of an isolated system remains constant or is conserved within a given frame of reference.
Information for Manufacturers and Retailers - Frequently asked questions about the Plastic Bag Ban Article 27 Title 28 New York State Bag Waste Reduction Act. Therefore the quantity of mass is conserved over time. Sometimes it may seem that matter disappears during a science experiment but this law tells us that matter cannot magically appear or disappear it simply changes from one form to another. The number and type of atoms must be the same for both reactants and products.
Simply stated the law of conservation of mass means matter cannot be created or destroyed but it can change forms. The Law of Conservation of Matter says that the amount of matter stays the same even when matter changes form. In both examples the reactants and products. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction.
The mass can neither be created nor destroyed. Conservation of energy principle of physics according to which the energy of interacting bodies or particles in a closed system remains constant. The law of conservation of mass was created in 1789 by a French chemist Antoine Lavoisier. The Law of Conservation of Matter Conservation of Mass.
As it is a law we assume it to be true in all cases even if our observations dont. More about Bag Waste Reduction Law. The law requires that during any. The total mass stays the same during a chemical reaction.
Law of Conservation of Matter Lab Group Members. Newtons laws of motion that the total momentum remains constant in a system completely separated from external factors. Exact conservation laws include conservation of mass and energy conservation of linear momentum conservation of angular momentum and conservation of electric chargeThere are also many approximate. Bag Waste Reduction Law.
According to the second law the momentum of each changes at a rate equal. The law of conservation of matter states that in any given system that is closed to the transfer of matter the amount of matter in the system stays constant. Be specific in your procedures so another person or group could reproduce your investigation and gather the same data and observations. In classical physics such laws govern energy momentum angular.
Because the conservation of mass makes it impossible. To mix in with another substance. In chemistry the law is used to balance chemical equations. Report a Plastic Bag Law Violation - Use this form to inform DEC of violations of the plastic bag ban.

The Law Of Conservation Of Matter Mass Conservation Conservation Of Mass Law

What Is The Law Of Conservation Of Mass Chemistry For All Fuseschool Conservation Of Mass Chemistry Chemistry Education

Law Of Conservation Of Matter Notes Interactive Science Notebook Interactive Science Science Notebook
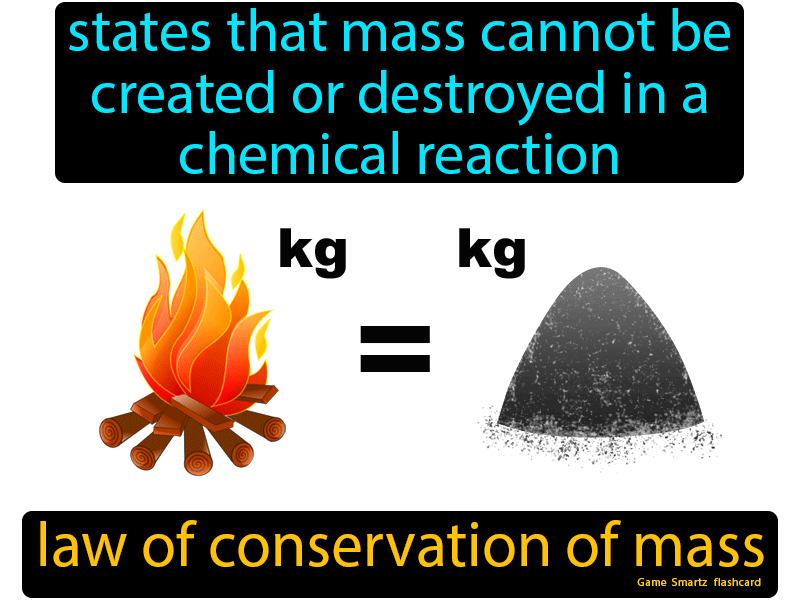
Law Of Conservation Of Mass Easy Science Conservation Of Mass Mass Activities Teaching Matter

Law Of Conservation Of Mass Cbse 9 Youtube Conservation Of Mass How To Memorize Things Chemical And Physical Changes
Posting Komentar untuk "what is the law of conservation of matter"